By now we know what was electricity and magnetism and the
connection but how do we quantify such an invisible force?.
How strong is strong?

Wilhelm Eduard Weber: German Doctor. 1804 – 1891
First use of c for speed of light
Work on magnetism
Electrodynamometer
Telegraphy
His work:
Mr Weber (I call him doctor induction) created a platform for us to quantify magnetic fields.
He made it possible for scientists and electricians alike to understand the
relationship between electricity and magnetism.
Like his hand rule:
***This is a slight modification from the
left-hand rule we already know. As soon as you get it - you will never
forget it.
Easy one: Grab a coil with the left hand, with the fingers pointing in
the direction of the coil's current flow then the the thumb will be pointing
to the north pole (the direction of the flux flow)
Complicated one: Grab a coil with the right hand and the fingers pointing
to CONVENTIONAL CURRENT FLOW then the thumb will be pointing to the north
pole of the electric field.
In order to visualise the invisible force we
needed a unit to quantify the electrical force field...He called it flux.
Why?
The word flux literally means: "to flow /
emanate from" and / or "an unsure state of change". This is exactly how
the electro-motive-force can be described.
And in his honour...flux is measured in Weber(Wb).
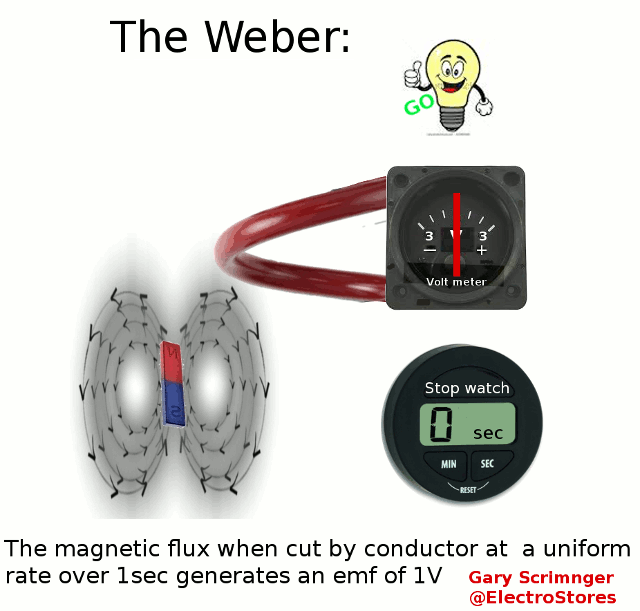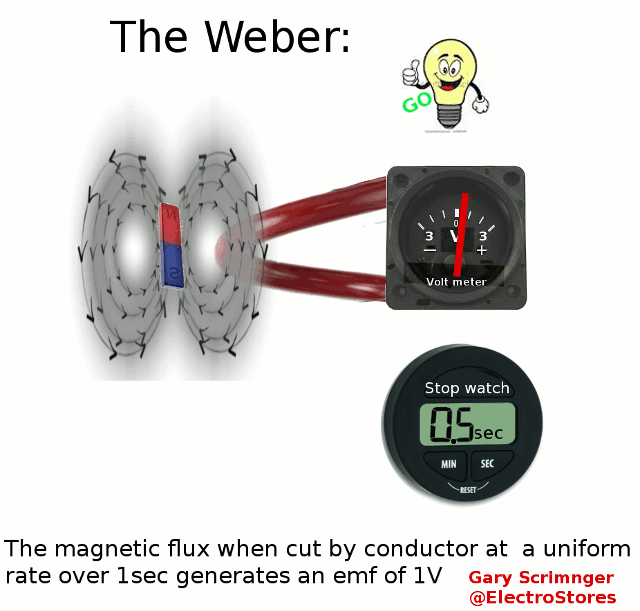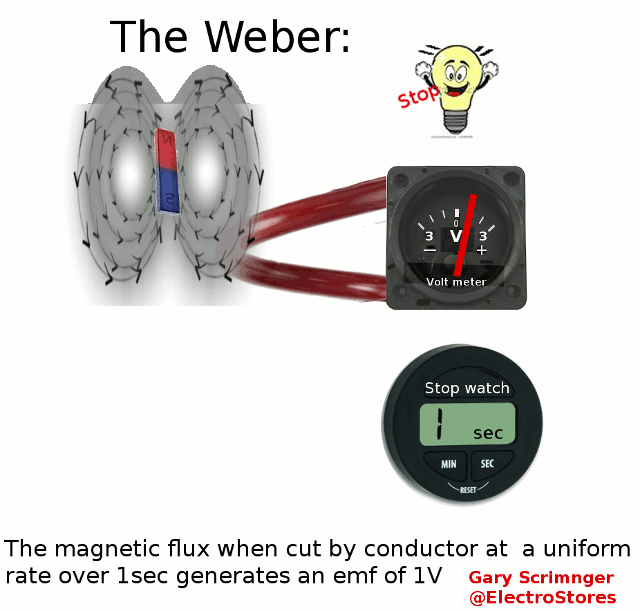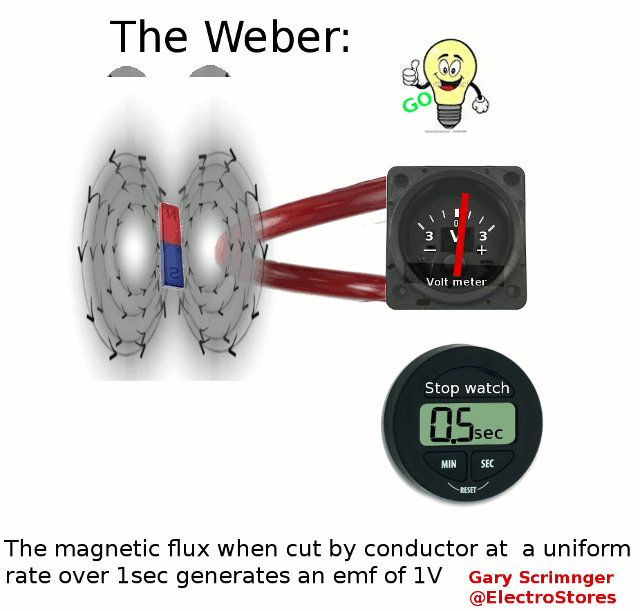
Definition:
A weber ( Φ or ΦB
)
is the magnetic flux
when linking a circuit of 1 turn produces an emf of 1Volt in it, when it
is is uniformly reduced to zero over 1sec.
---
---
To get there we have to start right at the
beginning:
The chemistry of all things...
But before we start - just by the way - since it is so intriguing: -
According to contemporaries - Matter is anything and everything that
is(exist).
The smallest identifiable part of matter is a molecule. So one can say
matter is made up of molecules.
The smallest identifiable part of a molecule is an atom. So one can say
molecules are made up of different atoms.
In the atom the electrons - like the planets of a galaxy - orbit a
nucleus (the sun).
How big are atoms - (0.1 to 0.5nm), i.e. a million times smaller
then diameter of a human hair (0.1mm) strand.
I also saw a reference where it is said that 99.999999999999% of an
atom's volume is empty! Nothing. One would wonder what keeps it all
together.
The electron inner orbits are spherical but as the distance grow the
pathways becomes less predictable.
The electron cloud has a radius 10,000 times greater than the nucleus.
The nucleus is made up of protons (and now neutrons).
From more than one resource it was said that a proton mass is
approximately 1800 times that of a neutron. The neutrons mass is slightly
higher than a proton.
Apparently and more spectacularly the electron, for hydrogen, are spinning
around the nucleus at 2.2 km/sec.
The arrangement and quantities of these elements
(electrons/protons/neutrons) determine ALL chemical elements. For the
chemists this is their bread and butter.
And this is where it gets interesting for the electrician: The
electrons are negatively charged and the protons are positively charged. The
neutron don't want to be part of the game, they are "neutral".
Electrostatics:
At atomic level the movement of the electrons makes up the
characteristics of the different material such as when there is equals
electrons and protons it carries no charge - the natural state of materials
- unless it is a magnet!
The nucleus is held together by the tight pull of what is known to
chemists and physicists as the "strong force."
On the other hand the the negative electrons is attracted to the nucleus
(opposites attract) and nucleus is known as the
electrostatic force.
This effect of opposites attract and similar charges repel each other is
referred to as the First Law of Electrostatics.
For some materials - such as copper - An applied emf will dislodge
electrons in a specific direction from atom to atom and this is our flow of electricity and
from this various other forms of energies can be derived.
What is an electrical charge?
When I found this information it just blew me away...it is not the
norm in the educational system... yet I believe it is the "more truer"
version of the definition of units and measurements.
An object’s electrical charge is determined by the number of
electrons that the object has gained or lost. Because such a large
number of electrons move, a unit called the "coulomb" is used to
indicate the charge.
One coulomb is equal to 6.28 x 10e18(billion, billion) electrons.
For example, if an object gains one coulomb of negative charge, it
has gained 6,280,000,000,000,000,000 extra electrons.
More conventionally : A volt is defined as a difference of potential
causing one coulomb of current to do one joule of work.
A volt is also defined as that amount of force required to force one
ampere of current through one ohm of resistance.